The projects these companies are working on can be organized into three distinct groups: Today’s graphics provide an in-depth look at who’s in the innovation race to defeat the virus, and they come to us courtesy of Artis Ventures, a venture capital firm focused on life sciences and tech investments. Editor’s note: R&D is moving fast on COVID-19, and the situation is quite fluid. While today’s post is believed to be an accurate snapshot of all innovations and developments listed by WHO and FDA as of March 30, 2020, it is possible that more data will become available.
Knowledge is Power
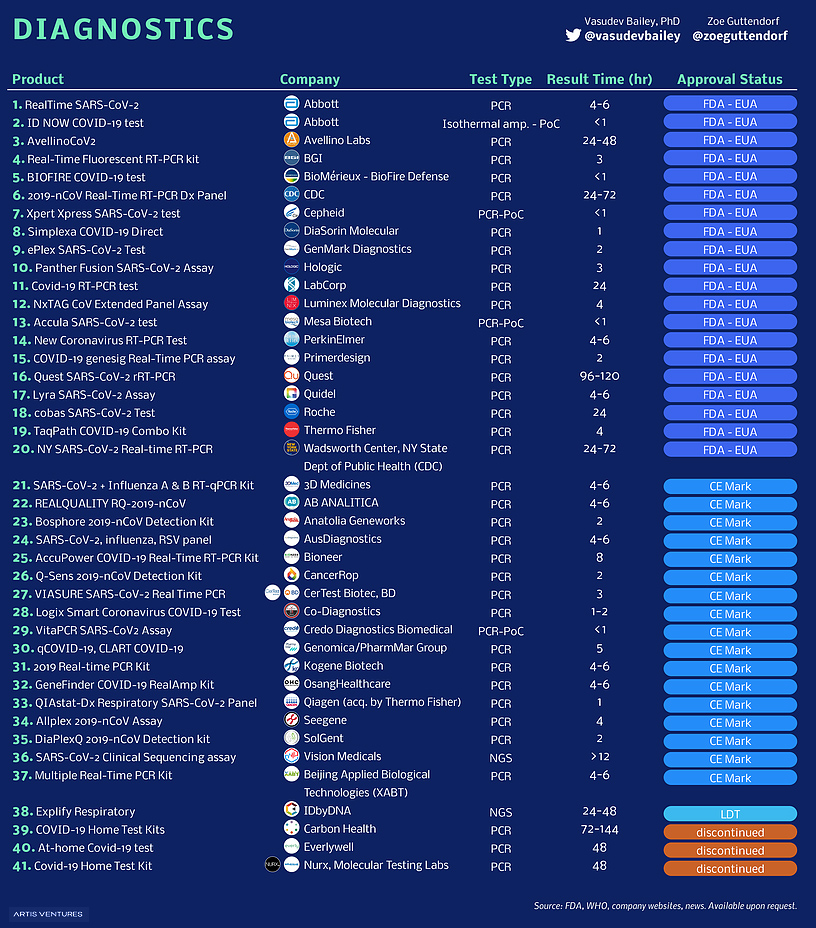
Testing rates during this pandemic have been a point of contention. Without widespread testing, it has been tough to accurately track the spread of the virus, as well as pin down important metrics such as infectiousness and mortality rates. Inexpensive test kits that offer quick results will be key to curbing the outbreak. Here are the companies and institutions developing new tests for COVID-19:
The ultimate aim of companies like Abbott and BioFire Defense is to create a test that can produce accurate results in as little as a few minutes.
In the Trenches With Coronavirus
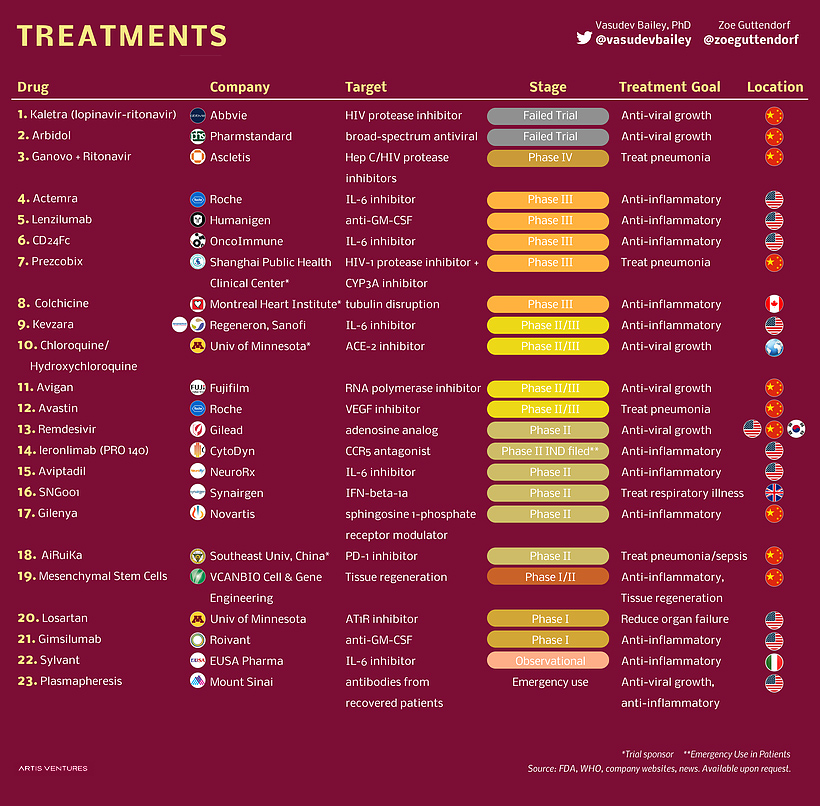
While the majority of people infected with COVID-19 only experience minor symptoms, the disease can cause severe issues in some cases – even resulting in death. Most of the forms of treatment being pursued fall into one of two categories: Here are the companies and institutions developing new treatment options for COVID-19:
A wide range of players are in the race to develop treatments related to COVID-19. Pharma and healthcare companies are in the mix, as well as universities and institutes. One surprising name on the list is Fujifilm. The Japanese company’s stock recently shot up on the news that Avigan, a decades-old flu drug developed through Fujifilm’s healthcare subsidiary, might be effective at helping coronavirus patients recover. The Japanese government’s stockpile of the drug is reportedly enough to treat two million people.
Vaccine
The progress that is perhaps being watched the closest by the general public is the development of a COVID-19 vaccine. Creating a safe vaccine for a new illness is no easy feat. Thankfully, rapid progress is being made for a variety of reasons, including China’s efforts to sequence the genetic material of Sars-CoV-2 and to share that information with research groups around the world. Another factor contributing to the unprecedented speed of development is the fact that coronaviruses were already on the radar of health science researchers. Both SARS and MERS were caused by coronaviruses, and even though vaccines were shelved once those outbreaks were contained, learnings can still be applied to defeating COVID-19.
One of the most promising leads on a COVID-19 vaccine is mRNA-1273. This vaccine, developed by Moderna Therapeutics, is being developed with extreme urgency, skipping straight into human trials before it was even tested in animals. If all goes well with the trials currently underway in Washington State, the company hopes to have an early version of the vaccine ready by fall 2020. The earliest versions of the vaccine would be made available to at-risk groups such as healthcare workers. Further down the pipeline are 15 types of subunit vaccines. This method of vaccination uses a fragment of a pathogen, typically a surface protein, to trigger an immune response, teaching the body’s immune system how to fight off the disease without actually introducing live pathogens.
No Clear Finish Line
Unfortunately, there is no silver bullet for solving this pandemic. A likely scenario is that teams of researchers around the world will come up with solutions that will incrementally help stop the spread of the virus, mitigate symptoms for those infected, and help lower the overall death toll. As well, early solutions rushed to market will need to be refined over the coming months. We can only hope that the hard lessons learned from fighting COVID-19 will help stop a future outbreak in its tracks before it becomes a pandemic. For now, those of us on the sideline can only do our best to flatten the curve. on The leading options for preventing infection include social distancing, mask-wearing, and vaccination. They are still recommended during the upsurge of the coronavirus’s latest mutation, the Omicron variant. But in December 2021, The United States Food and Drug Administration (USDA) granted Emergency Use Authorization to two experimental pills for the treatment of new COVID-19 cases. These medications, one made by Pfizer and the other by Merck & Co., hope to contribute to the fight against the coronavirus and its variants. Alongside vaccinations, they may help to curb extreme cases of COVID-19 by reducing the need for hospitalization. Despite tackling the same disease, vaccines and pills work differently:
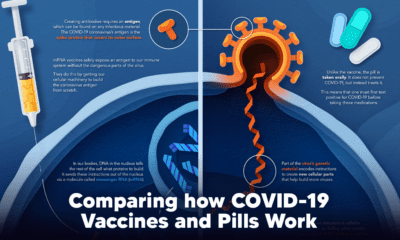
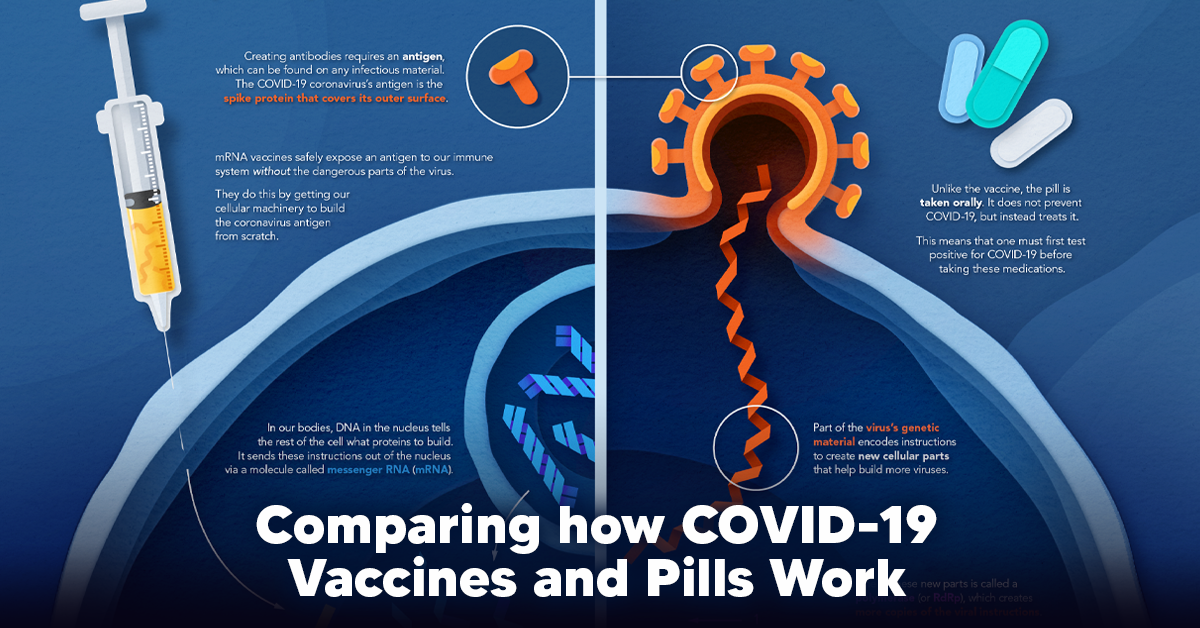
How a Vaccine Helps Prevent COVID-19
The main purpose of a vaccine is to prewarn the body of a potential COVID-19 infection by creating antibodies that target and destroy the coronavirus. In order to do this, the immune system needs an antigen. It’s difficult to do this risk-free since all antigens exist directly on a virus. Luckily, vaccines safely expose antigens to our immune systems without the dangerous parts of the virus. In the case of COVID-19, the coronavirus’s antigen is the spike protein that covers its outer surface. Vaccines inject antigen-building instructions* and use our own cellular machinery to build the coronavirus antigen from scratch. When exposed to the spike protein, the immune system begins to assemble antigen-specific antibodies. These antibodies wait for the opportunity to attack the real spike protein when a coronavirus enters the body. Since antibodies decrease over time, booster immunizations help to maintain a strong line of defense. *While different vaccine technologies exist, they all do a similar thing: introduce an antigen and build a stronger immune system.
How COVID Antiviral Pills Work
Antiviral pills, unlike vaccines, are not a preventative strategy. Instead, they treat an infected individual experiencing symptoms from the virus. Two drugs are now entering the market. Merck & Co.’s Lagevrio®, composed of one molecule, and Pfizer’s Paxlovid®, composed of two. These medications disrupt specific processes in the viral assembly line to choke the virus’s ability to replicate.
The Mechanism of Molnupiravir
RNA-dependent RNA Polymerase (RdRp) is a cellular component that works similar to a photocopying machine for the virus’s genetic instructions. An infected host cell is forced to produce RdRp, which starts generating more copies of the virus’s RNA. Molnupiravir, developed by Merck & Co., is a polymerase inhibitor. It inserts itself into the viral instructions that RdRp is copying, jumbling the contents. The RdRp then produces junk.
The Mechanism of Nirmatrelvir + Ritonavir
A replicating virus makes proteins necessary for its survival in a large, clumped mass called a polyprotein. A cellular component called a protease cuts a virus’s polyprotein into smaller, workable pieces. Pfizer’s antiviral medication is a protease inhibitor made of two pills: With a faulty polymerase or a large, unusable polyprotein, antiviral medications make it difficult for the coronavirus to replicate. If treated early enough, they can lessen the virus’s impact on the body.
The Future of COVID Antiviral Pills and Medications
Antiviral medications seem to have a bright future ahead of them. COVID-19 antivirals are based on early research done on coronaviruses from the 2002-04 SARS-CoV and the 2012 MERS-CoV outbreaks. Current breakthroughs in this technology may pave the way for better pharmaceuticals in the future. One half of Pfizer’s medication, ritonavir, currently treats many other viruses including HIV/AIDS. Gilead Science is currently developing oral derivatives of remdesivir, another polymerase inhibitor currently only offered to inpatients in the United States. More coronavirus antivirals are currently in the pipeline, offering a glimpse of control on the looming presence of COVID-19. Author’s Note: The medical information in this article is an information resource only, and is not to be used or relied on for any diagnostic or treatment purposes. Please talk to your doctor before undergoing any treatment for COVID-19. If you become sick and believe you may have symptoms of COVID-19, please follow the CDC guidelines.