Social distancing, defined as measures taken to reduce physical contact, is the first line of defense for containing an infectious disease like COVID-19. That’s because these infections spread when people cough, sneeze, or touch surfaces on which the virus resides. To help us grasp the impact these measures can actually have, today’s infographic illustrates how a reduction in social exposure can theoretically contain the spread of infection.
Theoretical Potential
The calculations used to create today’s infographic come from Signer Laboratory, a stem cell research lab located in the Moores Cancer Center at the University of California San Diego. Using a summation formula makes it possible to estimate the number of new infections over a 30 day period, across three scenarios. *For estimations only. It is not possible to infect only a fraction of another person. To arrive at the figures reported above, Robert A.J. Signer, Ph.D., and his team made a number of key assumptions. First, they estimated the basic reproduction number (R0) of COVID-19 to be 2.5, a figure supported by recent research. This means that, on average, an infected individual will spread the disease to 2.5 other people. Next, they assumed that an infected individual will unknowingly spread COVID-19 over the median five day incubation period. After this period, the individual will begin to develop symptoms, immediately self quarantine, and no longer pose a threat. Finally, they assumed a direct linear correlation between social interactions and R0. This means that when an infected person reduces their physical contact with others by 50%, they also spread the disease by an amount 50% less.
Timing is Everything
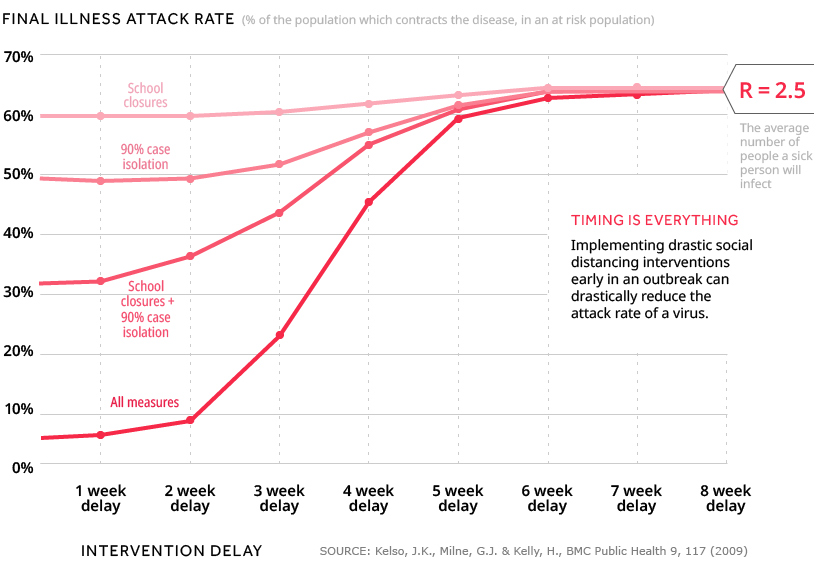
While the figures above are the results of mathematical estimations, researchers have actually studied social distancing from a variety of angles. One study used simulations to determine the magnitude and timing of social distancing measures required to prevent a pandemic. The distancing measures simulated were: The results, for a community of 30,000 people and an epidemic with R=2.5, are charted below. We can define the final illness attack rate as the share of people from an at risk population who ultimately catch the disease.
Results showed that when no action was taken, 65% of the population contracted the disease. However, if a combination of all four distancing measures were implemented instead, the attack rates were:
45% (distancing begins after a 4 week delay) 21% (distancing begins after a 3 week delay) 7% (distancing begins after a 2 week delay)
What’s clear is that social distancing was significantly more effective when implemented with minimal delay—the final illness attack rate rose quickest beyond the third week. These findings draw a parallel to the visualizations in today’s infographic, which showed us just how quickly a disease can spread. Kelso, J.K., Milne, G.J. & Kelly, H., BMC Public Health 9, 117 (2009) We arrive at a similar conclusion when it comes to the types of distancing measures implemented. In the simulations, none of the four measures taken on their own were able to have a similar effect as when they were combined.
We All Have a Part to Play
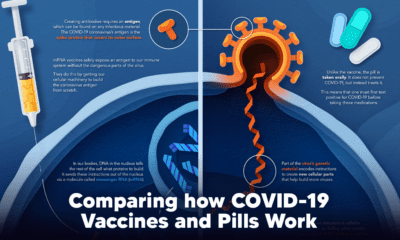
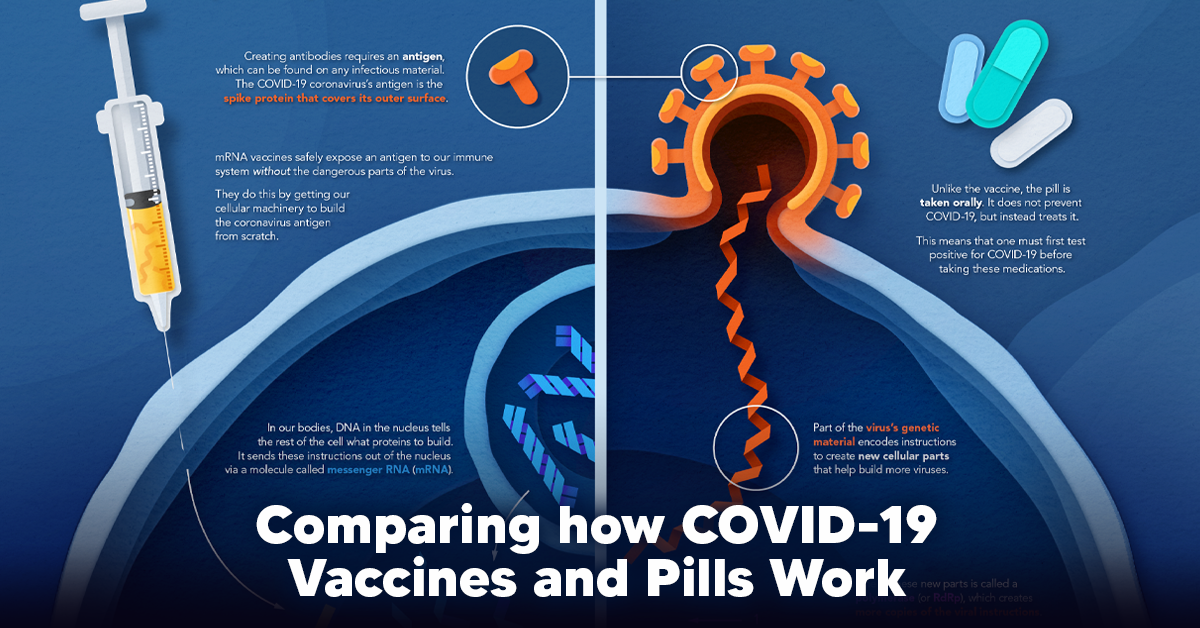
With the global number of COVID-19 cases still rising, many governments have issued quarantine orders and travel bans. The math supports these decisions—reducing our physical contact with others, even when we aren’t experiencing any symptoms, is crucial. Studies like the one summarized above also prove that taking action sooner, rather than later, can go a long way in reducing the spread of infection. The key takeaway from all of this? Social distancing is a powerful disease control tool, but only if we all participate. on The leading options for preventing infection include social distancing, mask-wearing, and vaccination. They are still recommended during the upsurge of the coronavirus’s latest mutation, the Omicron variant. But in December 2021, The United States Food and Drug Administration (USDA) granted Emergency Use Authorization to two experimental pills for the treatment of new COVID-19 cases. These medications, one made by Pfizer and the other by Merck & Co., hope to contribute to the fight against the coronavirus and its variants. Alongside vaccinations, they may help to curb extreme cases of COVID-19 by reducing the need for hospitalization. Despite tackling the same disease, vaccines and pills work differently:
How a Vaccine Helps Prevent COVID-19
The main purpose of a vaccine is to prewarn the body of a potential COVID-19 infection by creating antibodies that target and destroy the coronavirus. In order to do this, the immune system needs an antigen. It’s difficult to do this risk-free since all antigens exist directly on a virus. Luckily, vaccines safely expose antigens to our immune systems without the dangerous parts of the virus. In the case of COVID-19, the coronavirus’s antigen is the spike protein that covers its outer surface. Vaccines inject antigen-building instructions* and use our own cellular machinery to build the coronavirus antigen from scratch. When exposed to the spike protein, the immune system begins to assemble antigen-specific antibodies. These antibodies wait for the opportunity to attack the real spike protein when a coronavirus enters the body. Since antibodies decrease over time, booster immunizations help to maintain a strong line of defense. *While different vaccine technologies exist, they all do a similar thing: introduce an antigen and build a stronger immune system.
How COVID Antiviral Pills Work
Antiviral pills, unlike vaccines, are not a preventative strategy. Instead, they treat an infected individual experiencing symptoms from the virus. Two drugs are now entering the market. Merck & Co.’s Lagevrio®, composed of one molecule, and Pfizer’s Paxlovid®, composed of two. These medications disrupt specific processes in the viral assembly line to choke the virus’s ability to replicate.
The Mechanism of Molnupiravir
RNA-dependent RNA Polymerase (RdRp) is a cellular component that works similar to a photocopying machine for the virus’s genetic instructions. An infected host cell is forced to produce RdRp, which starts generating more copies of the virus’s RNA. Molnupiravir, developed by Merck & Co., is a polymerase inhibitor. It inserts itself into the viral instructions that RdRp is copying, jumbling the contents. The RdRp then produces junk.
The Mechanism of Nirmatrelvir + Ritonavir
A replicating virus makes proteins necessary for its survival in a large, clumped mass called a polyprotein. A cellular component called a protease cuts a virus’s polyprotein into smaller, workable pieces. Pfizer’s antiviral medication is a protease inhibitor made of two pills: With a faulty polymerase or a large, unusable polyprotein, antiviral medications make it difficult for the coronavirus to replicate. If treated early enough, they can lessen the virus’s impact on the body.
The Future of COVID Antiviral Pills and Medications
Antiviral medications seem to have a bright future ahead of them. COVID-19 antivirals are based on early research done on coronaviruses from the 2002-04 SARS-CoV and the 2012 MERS-CoV outbreaks. Current breakthroughs in this technology may pave the way for better pharmaceuticals in the future. One half of Pfizer’s medication, ritonavir, currently treats many other viruses including HIV/AIDS. Gilead Science is currently developing oral derivatives of remdesivir, another polymerase inhibitor currently only offered to inpatients in the United States. More coronavirus antivirals are currently in the pipeline, offering a glimpse of control on the looming presence of COVID-19. Author’s Note: The medical information in this article is an information resource only, and is not to be used or relied on for any diagnostic or treatment purposes. Please talk to your doctor before undergoing any treatment for COVID-19. If you become sick and believe you may have symptoms of COVID-19, please follow the CDC guidelines.